Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hidaka, Akihide; Yokoyama, Hiroya
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2017 (AWC 2017) (USB Flash Drive), p.29 - 42, 2017/09
no abstracts in English
 and Br
and Br and its effect on corrosion of a low-alloy steel
and its effect on corrosion of a low-alloy steelHata, Kuniki; Inoue, Hiroyuki*; Kojima, Takao*; Kasahara, Shigeki; Hanawa, Satoshi; Ueno, Fumiyoshi; Tsukada, Takashi; Iwase, Akihiro*
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2017 (AWC 2017) (USB Flash Drive), p.304 - 314, 2017/09
A model simulation of  radiolysis of mixed solutions of NaCl and NaBr was carried out. The simulation result agreed well with the experimental result, and Br
radiolysis of mixed solutions of NaCl and NaBr was carried out. The simulation result agreed well with the experimental result, and Br played an important role in determining the amounts of products from water radiolysis. The simulation result also showed that, in highly pure NaCl solutions, the steady-state concentration of a radolytic product, H
played an important role in determining the amounts of products from water radiolysis. The simulation result also showed that, in highly pure NaCl solutions, the steady-state concentration of a radolytic product, H O
O , was mainly controlled by three reactions (Cl
, was mainly controlled by three reactions (Cl +
+  OH
OH  ClOH
ClOH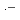 , ClOH
, ClOH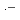
 Cl
Cl +
+  OH, and ClOH
OH, and ClOH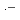 + H
+ H
 Cl
Cl + H
+ H O), which indicated that accurate evaluation of the rate constants of these reactions was very important in improving the radiolysis simulation of solutions containing Cl
O), which indicated that accurate evaluation of the rate constants of these reactions was very important in improving the radiolysis simulation of solutions containing Cl . An immersion test using a low-alloy steel, SQV2A, in the mixed solutions was also carried out under irradiation. The corrosion rate increased or decreased depending on the pH or the concentrations of the halide ions in a similar way to the change in concentration of H
. An immersion test using a low-alloy steel, SQV2A, in the mixed solutions was also carried out under irradiation. The corrosion rate increased or decreased depending on the pH or the concentrations of the halide ions in a similar way to the change in concentration of H O
O produced from water radiolysis, which is affected by the presence of Cl
produced from water radiolysis, which is affected by the presence of Cl and Br
and Br . However, at high pH values (
. However, at high pH values ( 12), the corrosion rate was almost zero even though the concentration of H
12), the corrosion rate was almost zero even though the concentration of H O
O was high. This could be attributed to enhancement of the passivity of test specimens at higher pH values.
was high. This could be attributed to enhancement of the passivity of test specimens at higher pH values.
Uchida, Shunsuke; Hanawa, Satoshi; Kysela, J.*; Lister, D. H.*
Power Plant Chemistry, 18(1), p.6 - 17, 2016/01
In order to establish reliable NPP operation, each plant requires its own unique optimal water chemistry control based on careful consideration of its system, materials and operational history. Electrochemistry is one of key issues that determine corrosion related problems, e.g., FAC. Based on the relationships among ECP, metal surface conditions and exposure time, a model to evaluate ECP and corrosion rate of steel was developed by coupling an electrochemical model and an oxide layer growth model. Major conclusions are as follows. (1) The effects of water chemistry improvement and mass transfer coefficients due to local flow velocity on FAC wall thinning rate and ECP could be evaluated with the proposed model. (2) The effects of H O
O and O
and O concentrations on ECP were evaluated with the model. Exposure time dependent ECPs were also explained as the effects of oxide film growth on the specimens. (3) Decreases in ECP due to neutron exposure were explained by radiation-induced diffusion in the oxide layers.
concentrations on ECP were evaluated with the model. Exposure time dependent ECPs were also explained as the effects of oxide film growth on the specimens. (3) Decreases in ECP due to neutron exposure were explained by radiation-induced diffusion in the oxide layers.
Uchida, Shunsuke; Hanawa, Satoshi; Lister, D. H.*
Power Plant Chemistry, 17(6), p.328 - 339, 2015/12
In nuclear power plants, radiation makes the relationship between structural materials and water chemistry much more complex than that in fossil fueled power plants. It is difficult to maintain safer and more reliable plant operation by controlling water chemistry based on only a restricted number of measured data. It is often required to control water chemistry with suitable assistance from computer models, which can extrapolate measured water chemistry parameters to those at the required locations and predict future trends of the interactions between structural materials and water chemistry. In the paper, water chemistry control based on parameters determined with plant simulation models and major computational models to be applied for water chemistry control are discussed.
Hanawa, Satoshi; Hata, Kuniki; Chimi, Yasuhiro; Nishiyama, Yutaka
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2013 (USB Flash Drive), 7 Pages, 2013/10
Water chemistry experiments will be carried out by using an in-pile loop newly installed in the JMTR. Concentrations of chemical species of O , H
, H and H
and H O
O are measured at the inlet and the outlet of the irradiation field. Electrochemical corrosion potential (ECP) at the irradiation field is also monitored. These experimental data will be obtained under wide range of experimental conditions such as absorption dose rate, H
are measured at the inlet and the outlet of the irradiation field. Electrochemical corrosion potential (ECP) at the irradiation field is also monitored. These experimental data will be obtained under wide range of experimental conditions such as absorption dose rate, H or O
or O concentration in the feeding water and water temperature. As a result of preliminary calculations, it became clear that the in-pile loop in the JMTR is capable for water chemistry experiment. Although the operation of the JMTR is being delayed because of the Tohoku district off the Pacific Ocean earthquake, construction of the loops and installation of the instrumentation for the loops have been carried out almost on schedule. The experiments will be started after JMTR restart.
concentration in the feeding water and water temperature. As a result of preliminary calculations, it became clear that the in-pile loop in the JMTR is capable for water chemistry experiment. Although the operation of the JMTR is being delayed because of the Tohoku district off the Pacific Ocean earthquake, construction of the loops and installation of the instrumentation for the loops have been carried out almost on schedule. The experiments will be started after JMTR restart.
 O
O
Sato, Tomonori; Kato, Chiaki; Ogi, Hirokazu*; Yamamoto, Masahiro; Ueno, Fumiyoshi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2013 (USB Flash Drive), 6 Pages, 2013/10
The hydrogen peroxide (H O
O ) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H
) generated by the water radiolysis in the reactor coolant under irradiated condition plays an important role for the stress corrosion cracking (SCC) in the boiling water reactors (BWRs). In this study, the corrosive condition in high temperature water containing H O
O was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H
was observed directly utilizing electrochemical impedance spectroscopy (EIS) in high temperature pure water. And the diffusion coefficient and thermal decomposition rate of H O
O at the 288
at the 288  C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H
C were estimated based on the obtained impedance responses. The reciprocal of the polarization resistance has linear relationship to the H O
O concentration. The estimated diffusion coefficient of H
concentration. The estimated diffusion coefficient of H O
O (D
(D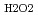 ) was 1.5
) was 1.5 10
10 cm
cm /s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H
/s when the thickness of the diffusion layer was assumed to be 0.05 cm. The thermal decomposition rate of H O
O in 288
in 288 C was 0.042 s
C was 0.042 s .
.
Hanawa, Satoshi; Chimi, Yasuhiro; Nishiyama, Yutaka; Nakamura, Takehiko
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009, p.221 - 225, 2009/10
Water chemistry in reactor core environment is of great interest in structural materials integrity evaluation, because it plays an important role in corrosion behaviours of the materials. In order to provide significant information on stress corrosion crack growth behaviours, water chemistry tests under well quantified corrosive condition will be performed. In order to perform the tests, a new test facility is being constructed in the Japan Material Testing Reactor (JMTR). The tests will be performed under various conditions by changing water chemistry of feeding water, dose rate and flow rate at the irradiation test section etc. parametrically. The construction of the test facility will be finished in 2012 to start the tests.
 value of hydroxyl radical at high temperatures
value of hydroxyl radical at high temperaturesMuroya, Yusa*; Takahashi, Hiroyuki*; Katsumura, Yosuke; Lin, M.; Kumagai, Yuta; Kudo, Hisaaki*
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009, p.71 - 75, 2009/10
Hydroxyl radical is one of the most important water decomposition products because it has strong oxidative property. However, its property higher than 200 C has not yet been clarified well. In this work, reaction kinetics and pK
C has not yet been clarified well. In this work, reaction kinetics and pK value of OH radical at elevated temperature over 300
value of OH radical at elevated temperature over 300 C have been investigated by means of nanosecond pulse radiolysis. Transient absorption spectra of formed radical and rate constants for the reaction with some solutes in aqueous solutions of benzoate, nitrobenzene, and carbonate etc., have been investigated. In addition, temperature dependent pK
C have been investigated by means of nanosecond pulse radiolysis. Transient absorption spectra of formed radical and rate constants for the reaction with some solutes in aqueous solutions of benzoate, nitrobenzene, and carbonate etc., have been investigated. In addition, temperature dependent pK value (
value ( OH/O
OH/O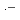 ) up to 300
) up to 300 C has also been evaluated by direct trace and indirect competitive reaction method.
C has also been evaluated by direct trace and indirect competitive reaction method.
 -ray irradiation and crevice-like shape on the corrosion of type 316L stainless steel in high-temperature water
-ray irradiation and crevice-like shape on the corrosion of type 316L stainless steel in high-temperature waterNakahara, Yukio; Kato, Chiaki; Yamamoto, Masahiro; Watanabe, Atsushi*; Fuse, Motomasa*
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009 (CD-ROM), p.226 - 231, 2009/10
The irradiation effect to high-temperature water in nuclear power plant has been regarded as one of important issues for preventing corrosion and stress corrosion cracking of plant materials. However, the effects of surface reaction and configurations of material on irradiated high-temperature water chemistry have been studied little because of the difficulty of measuring the environment. In this work, we have done a series of corrosion tests of Type 316L stainless steel in high-temperature water in order to estimate the effects of  -ray irradiation and crevice-like shape on the water chemistry. Test specimens immersed in high-temperature water of 288
-ray irradiation and crevice-like shape on the water chemistry. Test specimens immersed in high-temperature water of 288  C were
C were  -ray irradiated for 500 hours. The absorbed dose rate of
-ray irradiated for 500 hours. The absorbed dose rate of  -ray irradiation was estimated to be 30 kGy h
-ray irradiation was estimated to be 30 kGy h . The dimensions of the disk-like specimens were 16 mm in diameter by 0.5 mm in thickness. The surfaces of the specimens were mechanically finished with #800 emery paper. Sets of two specimens attached closely in order to simulate a crevice-like environment were also immersed. The surfaces of the specimens were analyzed using SEM, TEM, and laser Raman spectrometer. The results of surface analyses indicated that
. The dimensions of the disk-like specimens were 16 mm in diameter by 0.5 mm in thickness. The surfaces of the specimens were mechanically finished with #800 emery paper. Sets of two specimens attached closely in order to simulate a crevice-like environment were also immersed. The surfaces of the specimens were analyzed using SEM, TEM, and laser Raman spectrometer. The results of surface analyses indicated that  -ray irradiation enhanced the precipitation of iron oxide on the surface and the thickness of inner oxide layer became thicker by
-ray irradiation enhanced the precipitation of iron oxide on the surface and the thickness of inner oxide layer became thicker by  -ray irradiation.
-ray irradiation.  -ray irradiation also changed the morphology of oxide on the surface faced to the crevice-like environment.
-ray irradiation also changed the morphology of oxide on the surface faced to the crevice-like environment.
Lin, M.; Katsumura, Yosuke; Muroya, Yusa*; Yamashita, Shinichi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009 (CD-ROM), p.66 - 70, 2009/10
Supercritical water cooled reactor (SCWR) is expected to be one of the most promised next generation reactors (GenIV). Proper water chemistry control, in particular, the injection of H into the coolant to convert O
into the coolant to convert O and H
and H O
O into H
into H O by radiolytic processes, may represent the key to keep the integrity of the reactors. In recent years, studies on the radiolytic yields and rate constants up to supercritical conditions were performed. The experimental results show that the radiolytic yields at temperatures above 300
O by radiolytic processes, may represent the key to keep the integrity of the reactors. In recent years, studies on the radiolytic yields and rate constants up to supercritical conditions were performed. The experimental results show that the radiolytic yields at temperatures above 300 C do not follow linear relationship with temperature and there is a very significant density effects under supercritical conditions, especially around
C do not follow linear relationship with temperature and there is a very significant density effects under supercritical conditions, especially around 
 . The rate constants of many reactions do not follow linear Arrhenius relationship and there is also strong density dependence under supercritical conditions.
. The rate constants of many reactions do not follow linear Arrhenius relationship and there is also strong density dependence under supercritical conditions.
Sato, Tomonori; Noda, Kazuhiko*; Kato, Chiaki; Yamamoto, Masahiro; Nakano, Junichi; Tsukada, Takashi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2009 (CD-ROM), p.232 - 237, 2009/10
In this work, to clarify the electrochemical behaviors at the surfaces of stainless steels (SSs) in high temperature water containing hydrogen peroxide (H O
O ), the in-situ electrochemical impedance spectroscopy (EIS) of SSs exposed to high temperature water was carried out. The materials of test specimens were type 316L SS and type 304L SS. The range of the applied frequency in EIS was 100 kHz to 1 m or 10 mHz. The charge transfer resistance at the boundary between the oxide film and the base metal (R
), the in-situ electrochemical impedance spectroscopy (EIS) of SSs exposed to high temperature water was carried out. The materials of test specimens were type 316L SS and type 304L SS. The range of the applied frequency in EIS was 100 kHz to 1 m or 10 mHz. The charge transfer resistance at the boundary between the oxide film and the base metal (R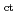 ) in oxygen (O
) in oxygen (O ) condition was larger than R
) condition was larger than R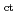 in H
in H O
O condition. This indicates that the corrosion rate of type 316L SS in high temperature water containing H
condition. This indicates that the corrosion rate of type 316L SS in high temperature water containing H O
O is larger than that in O
is larger than that in O contained water. The R
contained water. The R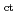 of type 316L SS was larger than that of type 304L SS in high temperature water containing H
of type 316L SS was larger than that of type 304L SS in high temperature water containing H O
O . This indicates that the corrosion resistance of type 316L SS is higher than that of type 304L SS.
. This indicates that the corrosion resistance of type 316L SS is higher than that of type 304L SS.
Sato, Tomonori; Uchida, Shunsuke; Tsukada, Takashi; Sato, Yoshiyuki*; Wada, Yoichi*; Ichigure, Kenkichi*
Proceedings of Symposium on Water Chemistry and Corrosion of Nuclear Power Plants in Asia, 2007 (CD-ROM), p.124 - 129, 2007/09
In the high temperature water containing H O
O , the dissolution of the oxide film formed at the surface of stainless steel was larger than that in O
, the dissolution of the oxide film formed at the surface of stainless steel was larger than that in O containing water. The oxide film at the surface of stainless steel formed in high temperature water is divided into inner and outer layers. The outer oxide layer consisted of the oxide particles. In this study, the changes in oxide particles were observed with changing exposure time. The obtained results are summarized in follows; (1) The particle density and size were changed by oxidant concentration. (2) In high [H
containing water. The oxide film at the surface of stainless steel formed in high temperature water is divided into inner and outer layers. The outer oxide layer consisted of the oxide particles. In this study, the changes in oxide particles were observed with changing exposure time. The obtained results are summarized in follows; (1) The particle density and size were changed by oxidant concentration. (2) In high [H O
O ] condition, the large hematite particle was formed, and it caused the high electric resistance of oxide film. (3) The large dissolution rate at high [H
] condition, the large hematite particle was formed, and it caused the high electric resistance of oxide film. (3) The large dissolution rate at high [H O
O ] resulted in a thinner oxide film with small particles and larger hematite particles. (4) The large hematite particle decreased the dissolution of the inner oxide film, while the removal of hematite particles caused the decrease of the total thickness of the oxide layer.
] resulted in a thinner oxide film with small particles and larger hematite particles. (4) The large hematite particle decreased the dissolution of the inner oxide film, while the removal of hematite particles caused the decrease of the total thickness of the oxide layer.
Tsukada, Takashi; Kaji, Yoshiyuki; Ugachi, Hirokazu; Nagata, Nobuaki*; Dozaki, Koji*; Takiguchi, Hideki*
Proceedings of Symposium on Water Chemistry and Corrosion of Nuclear Power Plants in Asia, 2005 (CD-ROM), 6 Pages, 2005/10
Irradiation assisted stress corrosion cracking (IASCC) is one of the significant concerns for the in-vessel stainless steel components of the aged light water reactors (LWRs). In general, IASCC can be reproduced on the materials irradiated over a certain threshold fluence level of fast neutron by the post-irradiation examinations (PIEs). It is, however, considered that the reproduced IASCC by PIEs must be carefully compared with the actual IASCC in nuclear power plants, because the actual IASCC occurs in the core under simultaneous effects of radiation, stress and high temperature water environment. Therefore, to confirm the effect of synergy, we have started to develop the test technique to carry out the in-pile IASCC tests at JMTR, Japan Materials Testing Reactor. In this paper, we describe the developed several techniques, especially control of loading on specimens, monitoring technique of crack initiation/growth and a result of mock-up in-pile SCC tests using thermally sensitized specimens.
Miwa, Yukio; Tsukada, Takashi
Proceedings of Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia 2003, p.301 - 306, 2003/00
Local composition change at grain boundaries due to radiation-induced segregation (RIS) followed by loss of corrosion resistance is considered to be a key mechanism on irradiation-assisted stress corrosion cracking (IASCC). However it is not clear that the local composition change induces the loss of corrosion resistance at grain boundaries, because RIS results in not only depletion of Cr but also enrichment of Ni and Si. This chemical composition change is different from that of thermally-sensitized stainless steels. In this study, experimental alloys were manufactured simulating the composition at grain boundaries of irradiated type 304 stainless steel and corrosion behavior of the experimental alloys was examined by weight loss measurement in 573 K water and anode polarization measurement in 1N sulfuric acid and 1mol/l sodium sulfate at 303 K. Following results were obtained: (1) In oxygenated water (DO=10ppm) at 573 K, weight loss increased with decreasing the concentration of Cr and did not depend on the concentration of Ni and Si. (2) Results of anode polarization measurements showed that alloys contained lower Cr and higher Ni and Si concentration exhibited lower corrosion potential in oxygenated, lower pH solution. In de-aerated solutions both sulfuric acid and sodium sulfate, however, there was a little influence of chemical composition on the corrosion potential.
Kameo, Yutaka; Aoki, Kazuhiro; ; Hirabayashi, Takakuni
Proc. of 1998 JAIF Int. Conf. on Water Chemistry in Nucl. Power Plants (Water Chemistry'98), p.571 - 574, 1998/00
no abstracts in English
; *; Hirabayashi, Takakuni; Aoki, Kazuhiro
Proc. of 1998 JAIF Int. Conf. on Water Chemistry in Nucl. Power Plants (Water Chemistry'98), p.566 - 570, 1998/00
no abstracts in English
Suwa, Takeshi; *; *; Tachikawa, Enzo
Proc. of 1991 JAIF Int. Conf. on Water Chemistry in Nuclear Power Plants: Water Chemistry,91, p.737 - 742, 1991/00
no abstracts in English